Obstructive coronary artery disease (myocardial infarction) is a great nightmare for mankind. In developed countries, as in Spain, it is the leading cause of mortality. According to data from the National Institute of Statistics, 26.4% of deaths in 2021 were due to cardiovascular diseases (INE data).
High cholesterol, especially the LDL fraction (bad cholesterol), is pointed out as the main responsible for this disease. Excess LDL cholesterol in the blood increases the likelihood that it will adhere to the inner walls of blood vessels, contributing to the formation of atheroma plaques, paving the way for what is called atherosclerosis. Consequently, the passageway narrows, blood flow is reduced and, depending on the severity, there may be a risk of rupture or obstruction of the blood vessel. If this occurs in the arteries of the heart, there is a risk of myocardial infarction (coronary atherosclerosis).
For this reason, high cholesterol has become public enemy number one. Paradoxically, no one questions that without cholesterol we die, and therefore cholesterol cannot be the only culprit. In fact, how can we explain the occurrence of myocardial infarction in increasingly younger adults, who also have no abnormalities in their cholesterol levels and adopt apparently healthy lifestyles? Yes, there is another culprit: homocysteine.
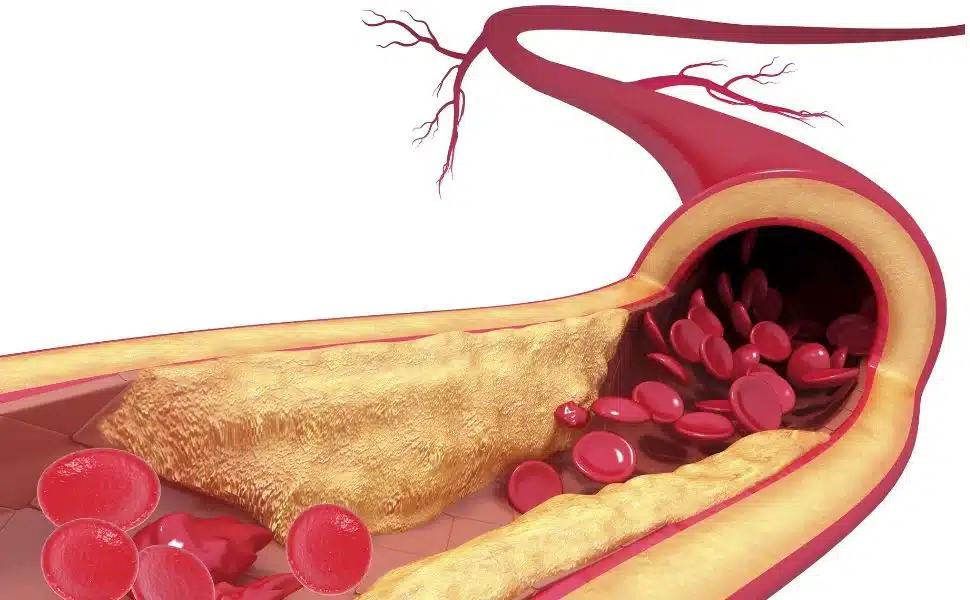
Figure 1: Illustration of atherosclerotic (atherosclerosis).
What is homocysteine?
Homocysteine is a by-product of the metabolism of methionine, an essential amino acid from dietary proteins, mainly animal proteins such as meat, milk and eggs. Methionine is metabolized to homocysteine mainly in the liver.
Where does homocysteine come from?
Most of the methionine in the diet is used for protein synthesis. However, a significant portion is used in the formation of S-adenosyl-L-methionine, a molecule with fundamental importance in a number of biochemical processes involving enzymatic methylation, an essential biochemical reaction that plays an important role in a wide range of biological processes such as gene expression, hormone, neurotransmitter and phospholipid synthesis.
Methionine is condensed with adenosine triphosphate (ATP) in cells, forming S-adenosyl-methionine. After donating the methyl group, S-adenosyl-methionine is transformed into S-adenosyl-homocysteine, which is hydrolyzed, generating adenosine and homocysteine that are released into the bloodstream.
Figure 3: Schematic diagram of the formation of homocysteine from methionine.
The resulting homocysteine is a very aggressive molecule for the body and needs to be metabolized (transformed) into another, less harmful substance. When homocysteine is found in high levels in the bloodstream, known as hyperhomocysteinemia, damage occurs to the inner walls of the blood vessels, which can lead to atherosclerosis due to the accumulation of atheromatous plaques and arterial stiffness 1. Another consequence of hyperhomocysteinemia is the alteration of the factors involved in blood coagulation, which can lead to the formation of clots that trigger thrombosis 1. Some studies also correlate elevated homocysteine levels in the blood with diseases such as Alzheimer’s, Parkinson’s, renal dysfunction and diabetes 2.
To date 3, 4, following the publication of several clinical and epidemiological studies, it has been shown that elevated plasma homocysteine levels are an independent risk factor for myocardial infarction, stroke and peripheral vascular disease. It is important to emphasize that an independent risk factor means that it is independent of other conventional cardiovascular disease factors such as elevated LDL cholesterol, elevated blood pressure, or smoking 5. This is important, because if you have elevated homocysteine and you also have another additional risk factor, the likelihood of cardiovascular disease is much higher.
Normal values of homocysteine in the blood
Currently, plasma homocysteine concentrations below 15 µmol/l in adults are considered to be non-risk and hyperhomocysteinemic patients are considered to be those with homocysteine levels above this value 6 :
| Classification | Concentration |
|---|---|
| Mild | 16 – 30 µmol/L |
| Moderate | 31 – 100 µmol/L |
| Severe | > 100 µmol/L |
Homocysteine metabolism
The metabolism of homocysteine in our body takes place mainly in the liver, where enzymes and vitamins B6 (pyridoxine), B12 (cobalamin) and 5-methyltetrahydrofolate, which is generated from folate (B9), are involved. These vitamins are very important because they act as enzyme cofactors, without which enzymes cannot perform their catalytic function.
How exactly do vitamins B6, B12 and B9 participate in homocysteine metabolism?
Homocysteine metabolism is carried out through 2 main routes: re-methylation and trans-sulfuration.
REMETHYLATION: If the body needs methionine, the remethylation route takes place, in which vitamins B12 and 5-methyltetrahydrofolate participate:
TRANSULFURATION: If there is sufficient methionine in the body, the trans-sulfuration pathway comes into action. In this case homocysteine combines irreversibly with serine to generate cystathionine. This route involves the enzyme cystathionine ß synthase and its cofactor vitamin B6. The next step is the conversion of cystathionine to cysteine.
In normal situations there should be very little free homocysteine left in the bloodstream. However, if the levels of vitamins B12, B6 and B9 in the liver are low, the liver is not able to metabolize homocysteine efficiently, leading to hyperhomocysteinemia, which can cause serious health problems, especially at the vascular level. In fact, individual or combined deficiency of these 3 vitamins is the most common cause of hyperhomocysteinemia 6, 8-10.
It should be noted that hyperhomocysteinemia can also be genetic in origin. Homocystinuria is a genetic disease that affects the function of enzymes involved in homocysteine metabolism. Persons with homocystinuria usually have very high plasma homocysteine concentrations, up to 20 times higher than normal values. The clinical manifestations of the disease include mental retardation, dislocation of the lens of the eye, skeletal disorders and premature atherosclerosis 11.
The importance of vitamins B6, B12 and folate (B9) for the maintenance of normal homocysteine levels.
In people with elevated homocysteine levels, the first therapeutic measure is a diet rich in vitamins B6, B12 and folate. According to the American Heart Association, this diet should provide 1.7 mg of vitamin B6, 2.4 µg of vitamin B12 and 400 µg of folate 12 per day. During pregnancy or lactation, these requirements increase, as well as in certain diseases such as renal failure.
According to the conclusions of the Framingham Heart Study, the daily intake of vitamin B6 and folate should be 3 mg and 400 µg of folate, respectively, for the prevention of hyperhomocysteinemia 13.
The Framingham Heart Study is a large observational research study initiated in 1948, and still ongoing, with the aim of investigating risk factors for cardiovascular disease.
Folates in the diet
In the European population, the average daily folate intake in men and women is 291 µg (197-326 µg) and 247 µg/day (168-320 µg), respectively 14. Since a significant percentage of the population does not meet the daily requirement for folic acid, a reasonable population strategy is to recommend an increase in the consumption of foods rich in this vitamin. It is important to keep in mind that folic acid is easily destroyed by excessive and prolonged heating, which will affect the amount of folate available from the diet.
Vitamin B12 and veganism
For people eating a balanced diet, vitamin B12 intake is usually adequate, except in vegans, who do not consume meat, fish or dairy products. As vitamin B12 is mainly present in foods of animal origin, strict vegans may have problems preventing the elevation of homocysteine in the blood 13 .
Atrophic gastritis, H. Pylori and vitamin B12
Another important aspect of vitamin B12 absorption is that vitamin B12 is bound to proteins in food. It takes the action of hydrochloric acid and stomach enzymes to release this vitamin B12. Therefore, people taking medications for heartburn, or for gastroesophageal reflux, people suffering from atrophic gastritis or Helicobacter pylori infection should have their vitamin B12 monitored. Age also tends to cause a decrease in stomach acid production.
Supplementation as a support
An alternative to ensure the intake of these vitamins and maintain normal homocysteine levels is supplementation. Indeed, several clinical trials have confirmed that folate (B9) supplementation, alone or in combination with vitamin B6 and B12, reduces plasma homocysteine concentration over a period of 2 to 6 weeks. 13 Treatment with these vitamin supplements has been shown to be effective, with minimal side effects and low cost. 13
Magnesium and homocysteine
Both DNA methylation (from S-adenosyl-L-methionine) and the remethylation of homocysteine to methionine require energy to occur. When we talk about energy at the cellular level, we are talking about ATP (adenosine triphosphate), and that is where magnesium comes in. ATP must be bound to magnesium to be biologically active. In other words, what we call ATP is actually ATP-Mg:
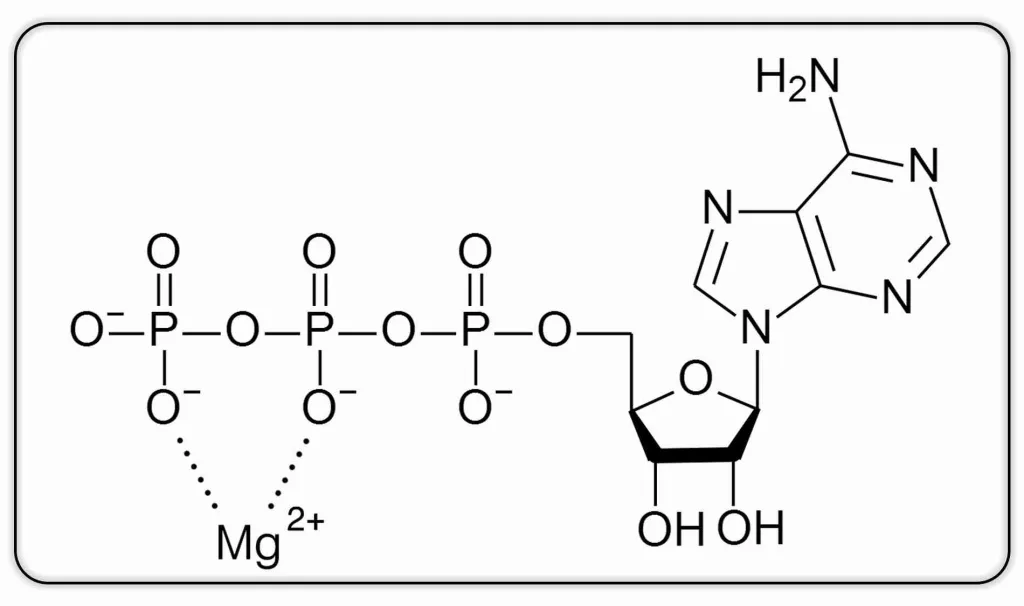
Figure 6:Homocysteine metabolism – Transulfuration: irreversible conversion of homocysteine to cysteine.
Some researchers hypothesize that magnesium may play an important role in methylation processes, which include both DNA methylation and the remethylation of homocysteine to methionine 15. According to these researchers, inadequate ATP-Mg production may contribute to increased homocysteine levels 15.
MAG-FUSION: Fusion of magnesium and the vitamins B6, B9 (folate), B12 and D3
MAG-FUSION is a Nutribiolite food supplement that precisely combines vitamins B6, B12 and folate in their most bioactive forms, magnesium in its most bioavailable form of magnesium citrate and vitamin D3 (cholecalciferol). It is a very complete food supplement, ideal for people who need supplementation in their diet to control their homocysteine levels, but not only. MAG-FUSION contains: 16
- Vitamins B6, B9 (folates) and B12 which contribute to normal homocysteine metabolism and help decrease tiredness and fatigue.
- Magnesium and vitamins B6, B9 (folates) and B12 that contribute to normal psychological function.
- Magnesium and vitamins B6 and B12 contribute to normal energy metabolism.
- Magnesium and vitamins B9 (folates), B12 and D that contribute to the cell division process.
- Vitamins B6, B9 (folates), B12 and D that contribute to the normal functioning of the immune system.
- Magnesium and vitamin D that contribute to the maintenance of bones and teeth in normal conditions, and to the normal functioning of muscles.
- Magnesium and vitamin B6 contribute to the normal functioning of the nervous system.
- Magnesium contributes to electrolyte balance and normal protein synthesis.
- Vitamin B6 contributes to normal cysteine synthesis, normal protein and glycogen metabolism, and helps regulate hormonal activity.
- Vitamin B9 (folates) contributes to normal amino acid synthesis and normal blood cell formation.
- Vitamin D contributes to the normal absorption and utilization of calcium and phosphorus and to the maintenance of normal blood calcium levels.
In the MAG-FUSION Nutribiolite formula, care has been taken to select its active ingredients in their most bioactive forms:
Vitamin B6 as Pyrodoxal-5′-phosphate.
Vitamin B6 is a group of three compounds called pyridoxine, pyridoxal and pyridoxamine. Pyridoxal-5′-phosphate, also known as P5P is the bioactive form of vitamin B6 that can be utilized directly by the body without conversion. Each of these forms of vitamin B6 perform unique functions in our body, but only the pyridoxal-5′-phosphate form has an enzyme cofactor function, participating in approximately 168 vital enzymatic processes in our body 17. In addition, P5P helps transport magnesium across cell membranes, which helps increase its absorption rate 18.
Vitamin B12 as methylcobalamin
There are two forms of vitamin B12 that you are likely to encounter in dietary supplements: methylcobalamin and cyanocobalamin. Cyanocobalamin is a synthetic form of vitamin B12, widely used in dietary supplements because it is cheaper. However, this form has a lower bioavailability, since the body first needs to get rid of the cyanide group after the action of the enzyme cyanocobalamin reductase. After the action of this enzyme, cyanocobalamin is finally converted into methylcobalamin or adenosylcobalamin, which are the two active forms of vitamin B12 19. Unlike cyanocobalamin, methylcobalamin is a natural form of vitamin B12 that can also be obtained from food sources such as fish, meat, eggs and milk.
Vitamin B9 (folate) as (6S)-5-methyltetrahydrofolate (5-MTHF)
Of all the available forms of folate, the one we are really interested in is the chemical form (6S)-5-methyltetrahydrofolate (5-MTHF). This is the bioactive form of vitamin B9. The other forms need to be digested in the intestine by the enzyme dihydrofolate reductase (DHFR) for conversion to 5-MTHF 20. In addition, in MAG-FUSION 5-MTHF is provided in the form of 5-MTHF glucosamine salt, also referred to as 4th generation folate or Quatrefolic®. According to laboratory studies, this patented form has an absorption 1.8 times higher than that of calcium (6S)-5-methyltetrahydrofolate 21, the chemical form of 5-MTHF used in other food supplements. As for its bioavailability, it has been verified in in vivo studies that its absorption is 3.1 times higher than that of folic acid. 21
The most common types of homocystinuria are those that affect the genes responsible for regulating the production of the CBS and MTHFR enzymes.11 In the case of deficiency in the production of the MTHFR enzyme, the body is unable to produce 5-methyltetrahydrofolate (5-MTHF), the bioactive form of folate in the body (Scheme 2). For this reason, MAG-FUSION presents folate directly in the form of 5-MTHF in its formula.
Vitamin D as cholecalciferol (D3)
MAG-FUSION features cholecalciferol or vitamin D3 as the form of vitamin D. This is the most effective form of vitamin D due to its more efficient conversion to calcifediol in the body. The other form of vitamin D is vitamin D2 or ergocalciferol. The potency of vitamin D2 is less than one-third that of vitamin D3 and its action in the body is shorter. 22
Magnesium as magnesium citrate
Magnesium in the chelated form of magnesium citrate, also known as 3:2 magnesium citrate, has a higher bioabsorption (better absorption by the body) than the more commonly used chemical forms of “inorganic salts” such as magnesium bicarbonate, chloride, oxide, phosphate or sulfate. 23, 24
Conclusion
Elevated homocysteine levels are strictly related to the formation of atheromas, the main cause of cardiovascular disease. In fact, numerous studies have clearly demonstrated that hyperhomocysteinemia is a predisposing factor for ischemic complications of atherosclerosis, venous thrombosis and pulmonary thromboembolism. 25, 26
Because of their direct relationship with folate, vitamin B12 and vitamin B6 status, the first therapeutic measure is a diet rich in these vitamins. If a daily intake of 400 µgof folate, 2.4 µgof vitamin B12 and 1.7-3 mg of vitamin B6 cannot be guaranteed, supplementation with food supplements is recommended. 27
If you have confirmed with your physician that your homocysteine levels are elevated, ask him/her whether supplementation with a food supplement based on vitamins B6, B12 and folate could be beneficial. In this case, we recommend MAG-FUSION, for the reasons mentioned above.
References
- Ganguly, P. and S.F. Alam, Role of homocysteine in the development of cardiovascular disease. Nutr J, 2015. 14: p. 6.
- Sharma, M., M. Tiwari, and R.K. Tiwari, Hyperhomocysteinemia: Impact on Neurodegenerative Diseases. Basic Clin Pharmacol Toxicol, 2015. 117(5): p. 287-96.
- Palma Reis, R., Homocysteinemia and vascular disease: Where we stand in 2022. Revista Portuguesa de Cardiologia, 2022. 41(10): p. 821-822.
- Porras, A.C., F.B. Vaca, and F.G. Sastre, Molecular basis of hyperhomocysteinemia. Quimica Clinica, 1998. 17(1): p. 5-18.
- Graham, I.M., et al., Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. Jama, 1997. 277(22): p. 1775-81.
- Varela-Moreiras, G., J.M. Escudero, and E. Alonso-Aperte, Homocysteine, related vitamins and lifestyles in the elderly: the SÉNECA study. Nutrición Hospitalaria, 2007. 22: p. 363-370.
- Morris, A.A., et al., Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J Inherit Metab Dis, 2017. 40(1): p. 49-74.
- Heijer, M.d., et al., Vitamin Supplementation Reduces Blood Homocysteine Levels. Arteriosclerosis, Thrombosis, and Vascular Biology, 1998. 18(3): p. 356-361.
- Selhub, J., et al., Vitamin Status and Intake as Primary Determinants of Homocysteinemia in an Elderly Population. JAMA, 1993. 270(22): p. 2693-2698.
- Ubbink, J.B., et al., Vitamin B-12, vitamin B-6, and folate nutritional status in men with hyperhomocysteinemia. Am J Clin Nutr, 1993. 57(1): p. 47-53.
- Przyrembel, H. Homocystinuria. 1982. Berlin, Heidelberg: Springer Berlin Heidelberg.
- Malinow, M.R., A.G. Bostom, and R.M. Krauss, Homocyst(e)ine, Diet, and Cardiovascular Diseases. Circulation, 1999. 99(1): p. 178-182.
- McCully, K.S., Homocysteine and vascular disease. Nat Med, 1996. 2(4): p. 386-9.
- G, V. and A. E., Ácido fólico y salud, in Fundación Española de la Nutrición. 1999.
- Józefczuk, J., et al, Homocysteine as a Diagnostic and Etiopathogenic Factor in Children with Autism Spectrum Disorder. J Med Food, 2017. 20(8): p. 744-749.
- Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health (Updating Regulation (EC) No 1924/2006), in Official Journal of the European Union. 2012.
- Spinneker, A., et al., Vitamin B6 status, deficiency and its consequences: an overview. Hospital Nutrition, 2007. 22: p. 7-24.
- Pouteau, E., et al., Superiority of magnesium and vitamin B6 over magnesium alone on severe stress in healthy adults with low magnesemia: A randomized, single-blind clinical trial. PLOS ONE, 2018. 13(12): p. e0208454.
- Paul, C. and D.M. Brady, Comparative Bioavailability and Utilization of Particular Forms of B(12) Supplements With Potential to Mitigate B(12)-related Genetic Polymorphisms. Integrative medicine (Encinitas, Calif.), 2017. 16(1): p. 42-49.
- Visentin, M., et al., The intestinal absorption of folates. Annual review of physiology, 2014. 76: p. 251-274.
- Miraglia, N., et al., Enhanced oral bioavailability of a novel folate salt: comparison with folic acid and a calcium folate salt in a pharmacokinetic study in rats. Minerva Gynecol, 2016. 68(2): p. 99-105.
- Armas, L.A., B.W. Hollis, and R.P. Heaney, Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab, 2004. 89(11): p. 5387-91.
- Coudray, C., et al., Study of magnesium bioavailability from ten organic and inorganic Mg salts in Mg-depleted rats using a stable isotope approach. Magnesium Research, 2005. 18(4): p. 215-223.
- Kappeler, D., et al., Higher bioavailability of magnesium citrate as compared to magnesium oxide shown by evaluation of urinary excretion and serum levels after single-dose administration in a randomized cross-over study. BMC Nutrition, 2017. 3(1): p. 7.
- Zinellu, A., et al., A systematic review and meta-analysis of homocysteine concentrations in chronic obstructive pulmonary disease. Clin Exp Med, 2022.
- Chrysant, S.G. and G.S. Chrysant, The current status of homocysteine as a risk factor for cardiovascular disease: a mini review. Expert Rev Cardiovasc Ther, 2018. 16(8): p. 559-565.
- Pintó Sala, X., Homocysteine as a cardiovascular risk factor. Medicina Integral, 2000. 36(5): p. 179-185.