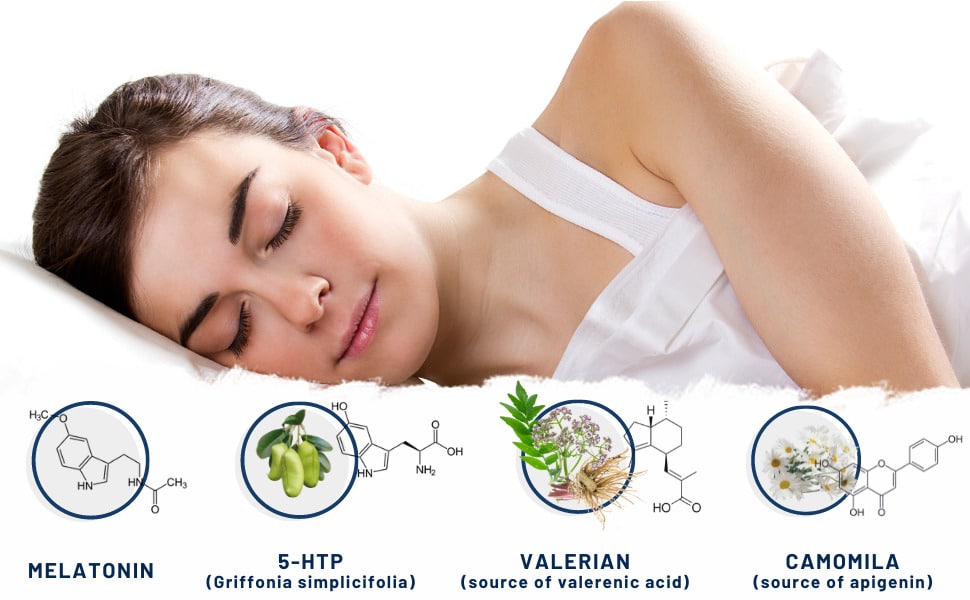
4 powerful ingredients to help improve your sleep
A key to a healthy life is getting a good night’s sleep. It is during sleep when our body performs its main restorative functions, such as tissue regeneration, muscle growth and protein synthesis. During this time, it is possible to replenish energy and regulate metabolism, essential factors for maintaining a healthy body and mind and preventing the onset of chronic diseases. Sleep is also important for appetite regulation, as it regulates stress-related hormones (cortisol), which helps control weight and blood glucose levels.
How many hours of sleep are enough for good health?
Table 1: Recommended hours of sleep according to age (1).
| Groups | Hours of sleep/day |
| Newly born | 14-17 |
| Babies | 12-16 |
| Children (1-2 years old) | 11-14 |
| Preschoolers (3-5 years old) | 10-13 |
| School-age children (6-12 years old) | 9-12 |
| Teens | 8-10 |
| Adults | 7-9 |
| Seniors (65+ years old) | 7-8 |
Unfortunately, at various times in our lives, sleeping this many hours and with the necessary quality is almost impossible and, especially depressing, when suffering from insomnia. Day-to-day stress and worries can negatively affect biological rhythms, making it difficult to sleep and decreasing its quality.
Benzodiazepines
A widely used resource for the treatment of severe sleep disorders and anxiety are psychoactive drugs based on benzodiazepines, such as Diazepam (Valium), Alprazolan (Xanax), Lorazepam (Ativan) or Estazolam (Prosom). These drugs must be used rationally and under strict medical prescription, since they generate dependency very easily. In fact, the use of benzodiazepines for a period of time of approximately 3 to 6 weeks, even using therapeutic doses, easily leads to a state of dependence (2).
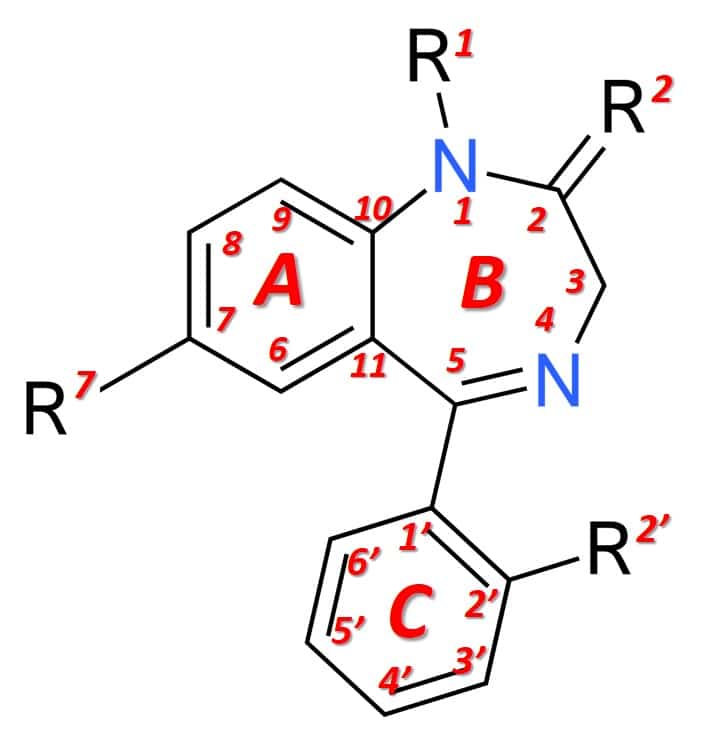
Structurally (figure 1), benzodiazepines are the result of the fusion of a benzene ring (ring A) with a 1,4-diazepine ring (ring B). Benzodiazepines that express anesthetic effects more markedly also have an aryl ring in position 5 (ring C), which increases their potency at the pharmacological level. The different benzodiazepines available on the market have different chemical groups in positions 1, 2, 5 or 7 and additionally in 2 ′, which modulate the pharmacological properties, the potency of the effect and the pharmacokinetic conditions (duration of effect, distribution, etc.) .
Figure 1: General structure of a benzodiazepine.
The mechanism of action of benzodiazepines.
GABA (γ-Aminobutyric acid) is the most important inhibitory neurotransmitter in the central nervous system. The function of GABA is to inhibit or reduce neuronal activity through its action on specific receptors called GABA-A receptors. These receptors are proteins located on the membranes of neurons in synaptic spaces (the space between a presynaptic and a postsynaptic neuron), where chemical synapse occurs. As a consequence, the inhibition of neuronal activity produces a feeling of calm, muscle relaxation and sedation, which plays an important role in behavior, cognition and the body’s response to stress (3). Due to their ability to bind to certain sites (alpha subunits) located on GABA-A receptors, benzodiazepines modulate the effect of GABA by acting as enhancers of its suppressive effect on neuronal activity. For this reason, benzodiazepines are responsible for causing anxiolytic (anxiety reducing), hypnotic (sleep inducing), sedative, anticonvulsant and muscle relaxant effects.
The public health problem generated by the use of benzodiazepines
The main problem associated with the use of benzodiazepines is their ability to generate addiction. One of the typical signs of the establishment of addiction in many patients is the appearance of withdrawal syndrome, caused after a sudden interruption of the chronic and prolonged intake of these substances. Typical withdrawal symptoms are: anxiety, panic attacks, hyperventilation, tremors, sleep disturbances, muscle spasms, anorexia, weight loss, visual disturbances, sweating, dysphoria, hypersensitivity to noise, seizures, hallucinations, and delusions. To further complicate the situation, in many European countries (4,5), benzodiazepines are often used in an uncontrolled manner by the population, giving rise to a serious public health problem that worries the authorities, since that addiction to this substance is difficult to manage therapeutically.
The pleasurable sensations that addictive drugs normally generate, and which are disastrously attractive to vulnerable people, occur when dopamine levels in the reward (or pleasure) area of the brain rise abruptly. These types of drugs interfere with the body’s natural mechanisms to control the flow of dopamine. More specifically, benzodiazepines weaken the action of a group of neurons called interneurons, which are found in the ventral tegmental area of the brain (VTA). Interneurons normally help prevent excessive dopamine levels by regulating the firing rates of dopamine-producing neurons. Therefore, when benzodiazepines limit the restrictive influence of interneurons, dopamine-producing neurons release more dopamine than normal (6).
Dopamine is the chemical that mediates pleasure in the brain. Its production takes place in pleasant situations and aims to stimulate the performance of pleasant activities, important for survival, such as food or sex. Some drugs such as opioids and benzodiazepines have the ability to artificially stimulate dopamine production in the brain, leading to imbalances in the natural reward/survival process.
Natural alternatives to benzodiazepines
Since the factors responsible for anxiety and insomnia are not expected to decrease, it is essential for the patient’s quality of life to find anxiolytic alternatives with less potential to induce adverse reactions. In this sense, there has been great interest on the part of the scientific community in the study of medicinal plants (7). Since ancient times, some plants have been used to treat insomnia and anxiety, and today the use of their extracts has gained increasing acceptance by the medical community (7). However, most medicinal plants lack chemical and pharmacological data and, in many cases, their active principles have not yet been identified or chemically studied. It is expected that with the advancement of scientific studies in the area of phytochemistry and phytomedicine, we will have a greater amount of natural resources to help in the treatment of this type of disorder.
Among the various medicinal plants known for their anxiolytic, sedative and relaxing properties, we can highlight kava-kava (Piper methysticum), passionflower (Passiflora incarnata), grifonia (Griffonia simpliciflia), valerian (Valeriana officinalis), chamomile (Matricaria recutita) and arnica (Galphimia glauca). These plants have been extensively studied and their most important active ingredients have been identified. Among those examples, kava-kava was no longer authorized as an ingredient in food supplements in Europe after 2002, due to suspected hepatotoxicity (8).
4Sleep: 4 powerful ingredients to help you sleep better
Aware of the need to offer safe alternatives to help people who suffer from insomnia and have difficulties falling asleep, Nutribiolite has developed 4Sleep, a food supplement that combines melatonin and the concentrated extracts of griffonia, valerian and chamomile. Each of these ingredients demonstrated, in various scientific research articles, important anxiolytic properties that help promote the biological processes associated with relaxation and falling asleep. The combination of these ingredients aims to obtain an additive effect that provides a progressive and long-lasting sleep-inducing effect. In the following chapters, we provide a brief summary of the properties of each of these 4Sleep ingredients.
Melatonina
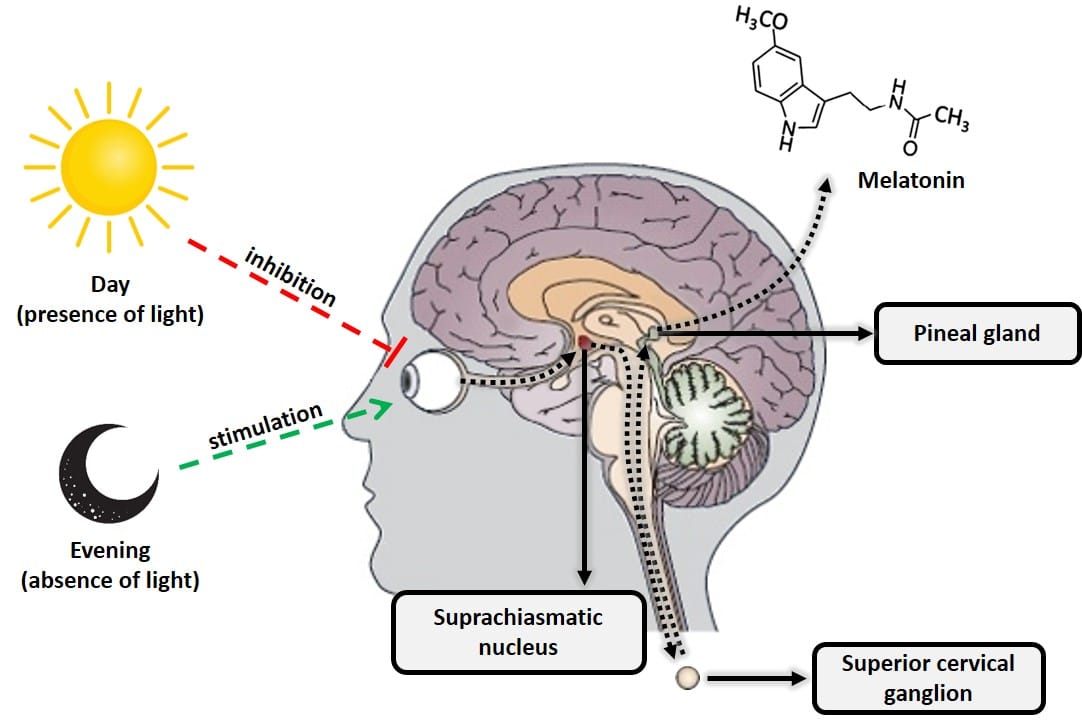
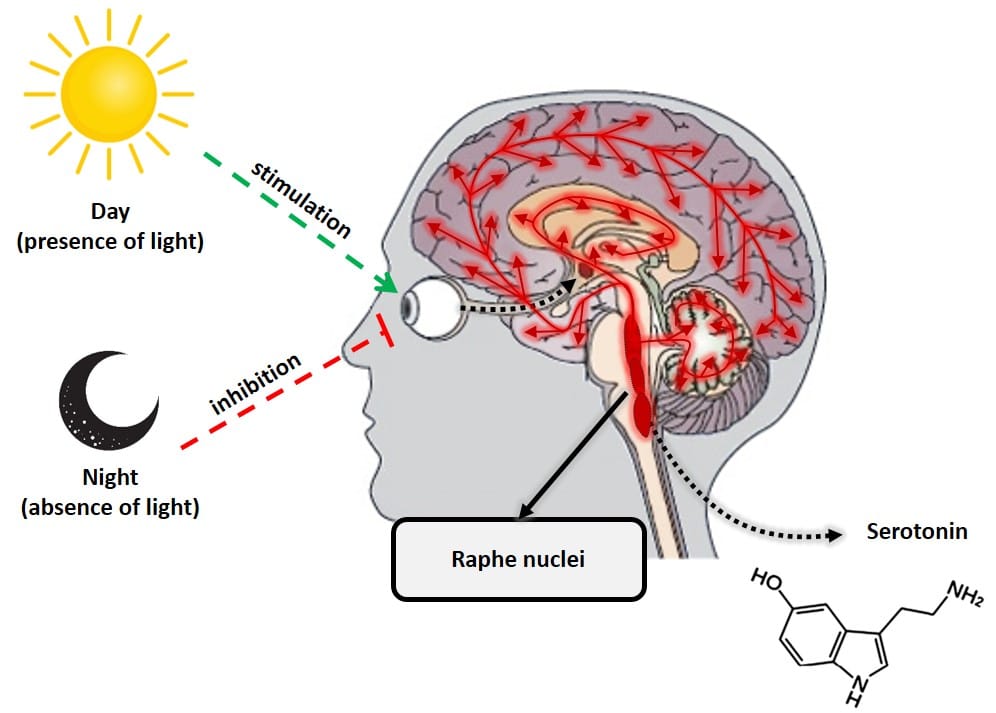
Melatonin, or N-acetyl-5-methoxytryptamine, is a hormone produced primarily by the pineal gland (located at the back of the brain) from the amino acid tryptophan and derived from another hormone, serotonin. Melatonin is extremely important for regulating the body’s biological clock (circadian cycle or sleep-wake cycle) and energy metabolism. This hormone is produced when the retina detects the absence of light, in such a way that its daily biosynthesis is precisely due to a rhythmic circadian production synchronized with the characteristic ambient light cycle of day and night. This daily rhythmic production is such that, in any species considered, the peak of production occurs at night. Another important characteristic of the functional neural system that regulates the synthesis of melatonin is that the light present in the environment at night can completely block (depending on its intensity and wavelength, mainly blue light of 480 nm), the synthesis of melatonin pineal (9). Therefore, the total absence of artificial light is important to fall asleep. Habits like using a smartphone or other electronic device with luminescent displays make it difficult to create the biological mechanisms that trigger the natural production of melatonin.
(click on the image to enlarge)
Figure 2: Diagram of the processes that inhibit and stimulate pineal melatonin production.
The amount of melatonin is not constant throughout life. Its production begins in babies from 3-4 months of age and its levels increase throughout childhood until reaching a maximum value between 8-10 years old. From there, the production of melatonin begins to decline, a process that will continue until the end of the life. When we reach 40-45 years old, the decrease in melatonin production becomes more abrupt. In people older than 70 years old, melatonin levels are already below 10% of the values typically seen in children between 8-10 years old (10).
According to several scientific studies, oral melatonin supplementation is beneficial and helps reduce the time it takes to fall asleep (11, 12), reduces jet-lag-related sleep disorders in night workers (13), helps with attention deficit hyperactivity disorder (ADHD) (14), with changes in the circadian rhythm of blind people (15) and with sleep problems related with menopause (16, 17). Melatonin readily crosses the blood-brain barrier and accumulates in the central nervous system at levels substantially higher than those found in the blood (18). In this sense, supplementation with (exogenous) melatonin is quite effective, especially in individuals with low levels of endogenous melatonin (19). Due to the easy accumulation of melatonin in the brain, it is not recommended to take high daily amounts of this substance, especially in older people, due to decreased liver and kidney function, as well as changes in body composition (retention of fat and water) that alter the pharmacokinetic properties of melatonin and increase the risk of adverse effects such as drowsiness the next day (20, 21).
Taking into account the need for a better risk/benefit ratio for European consumers, in 2001, at the request of the European Commission, the Panel on Dietetic Products, Nutrition and Allergies of the European Food Safety Authority (EFSA) issued a scientific opinion about the beneficial properties of melatonin for sleep and what amounts would be ideal to obtain these benefits. The panel considered several placebo-controlled meta-analysis studies in people without sleep problems, in people with primary sleep disorders, and in people with insomnia (26). Based on this scientific review, EFSA concluded that 1 mg of melatonin consumed before bed effectively helps recovery from sleep (22–25). As a result, several European countries have established limits on the maximum authorized daily dose of melatonin in food supplements, for example: Spain (1.9 mg), Portugal (1.9 mg), Greece (3.0 mg), France ( 2.0 mg), the Netherlands (0.3 mg) and Italy (1 mg). Based on these values, melatonin is considered a drug and its sale is controlled by medical prescription and only indicated for people diagnosed clinically with endogenous melatonin deficiency.
Based on the EFSA scientific report and complying with the regulations of all the European countries in which Nutribiolite markets its products, 4Sleep incorporates exactly 1 mg of melatonin in its formula. In fact, a larger amount (up to 1.9 mg) of this active ingredient is unnecessary, according to several meta-analysis studies (22–25). In addition, the process of falling asleep also depends on other relaxation mechanisms that are triggered in the central nervous system, which is why 4Sleep is based on the combination of melatonin with three other active principles obtained from the medicinal plants grifonia, valerian and chamomile that we describe below.
Griffonia: source of 5-HTP, precursor of serotonin and melatonin
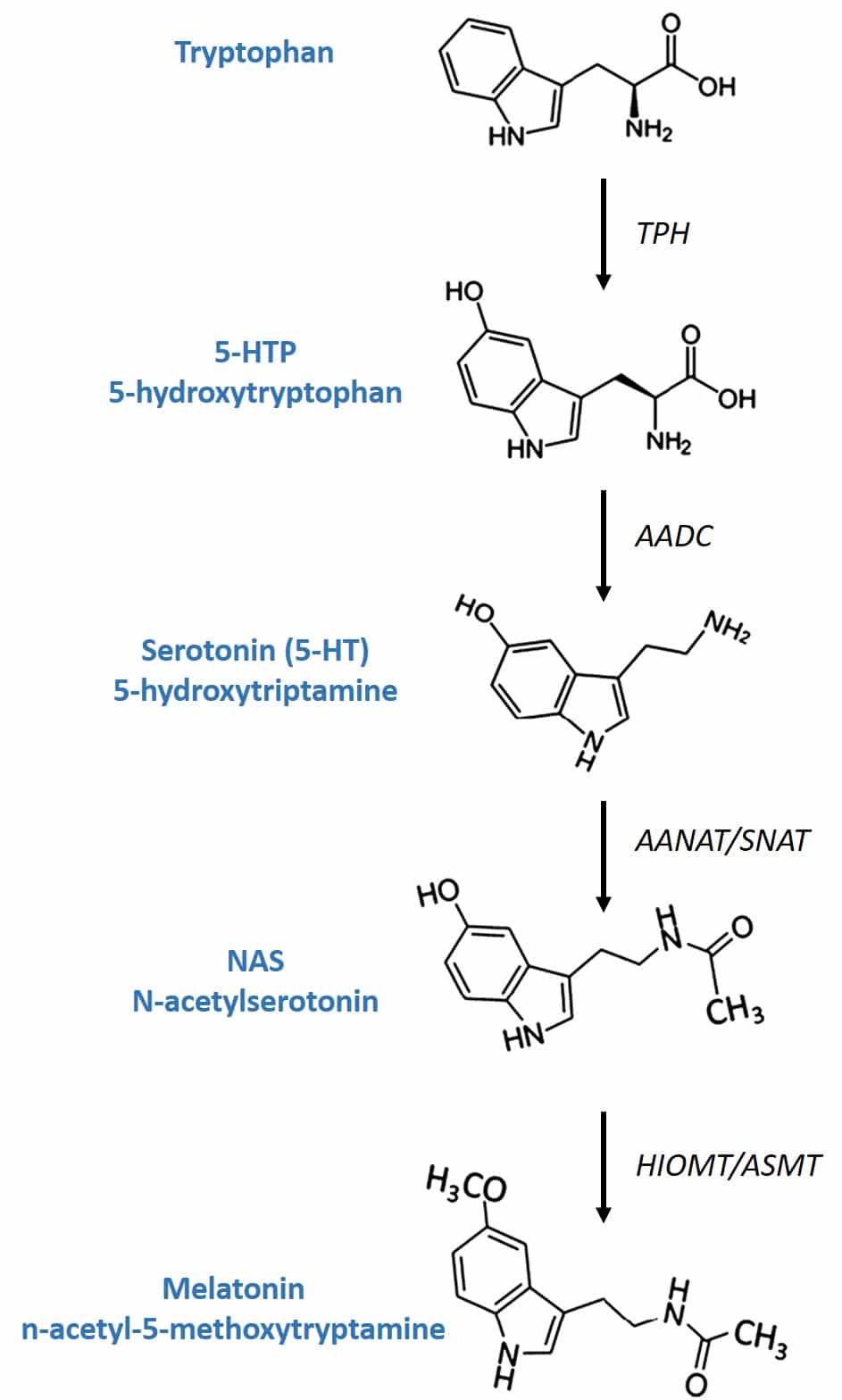
Another active ingredient present in the 4Sleep formula is L-5-hydroxytryptophan, also known as 5-HTP, an amino acid precursor and intermediate in the biosynthesis of the neurotransmitters serotonin and melatonin (figure 3). The existence of 5-HTP in nature is not only limited to mammals, such as humans, but is also produced by plants, fungi, and microbes. The seed of the grifonia (Griffonia simplicifolia) is one of the richest plant sources of 5-HTP, which has led to the development of very robust methods both for the production of concentrated extracts and for their analytical characterization. Consequently, both pharmaceutical laboratories and food supplement manufacturing laboratories currently have at their disposal extracts of griffonia standardized in terms of 5-HTP of excellent quality (27). 4Sleep uses an extract of highly concentrated in 5-HTP (> 98%), determined by high performance liquid chromatography (HPLC).
(click on the image to enlarge)
Figure 3: Melatonin biosynthesis.
Conversion of 5-HTP to serotonin in the body takes place through the enzyme AADC (aromatic L-amino acid decarboxylase). Its conversion to melatonin involves two enzymes that are produced by the pineal gland after being induced by the retina due to lack of light: the enzyme SNAT (serotonin-N-acetyltransferase) converts serotonin into N-acetylserotonin and the enzyme (HIOMT) hydroxyindole-O-methyltrasferase transfers a methyl group of S-adenosylmethionine to the 5-hydroxyl of N-acetylserotonin, giving rise to melatonin.
Serotonin and melatonin are almost literally day and night in hormonal terms. These two hormones do the opposite job, but in complete harmony, to keep the body in balance. Serotonin, known as the “happiness hormone,” is a neurotransmitter that plays a key role in regulating almost all central nervous system functions, such as mood, anxiety, stress, hunger, cognition and sexual behavior. Unlike melatonin, serotonin has its maximum production during the day, when the retina detects the presence of light. Its production takes place mainly in an area of the brain called the raphe nuclei.
(click on the image to enlarge)
Figure 3: Serotonin production by raphe nuclei.
The role of 5-HTP in 4Sleep formula is to provide a secondary source of melatonin, which will be gradually produced via serotonin during the night. In fact, several scientific studies have found that the administration of 5-HTP, in addition to increasing serotonin levels, also produced an increase in melatonin levels (28).
Despite acting as a source of 5-HTP in the body, supplementation with L-tryptophan, rather than 5-HTP, has disadvantages. This is because the body also uses L-tryptophan for other purposes, such as protein formation and the production of niacin from kynurenine. In addition, 5-HTP crosses the blood-brain barrier much more easily and is converted to serotonin more quickly (higher bioavailability) (29).
Chamomile
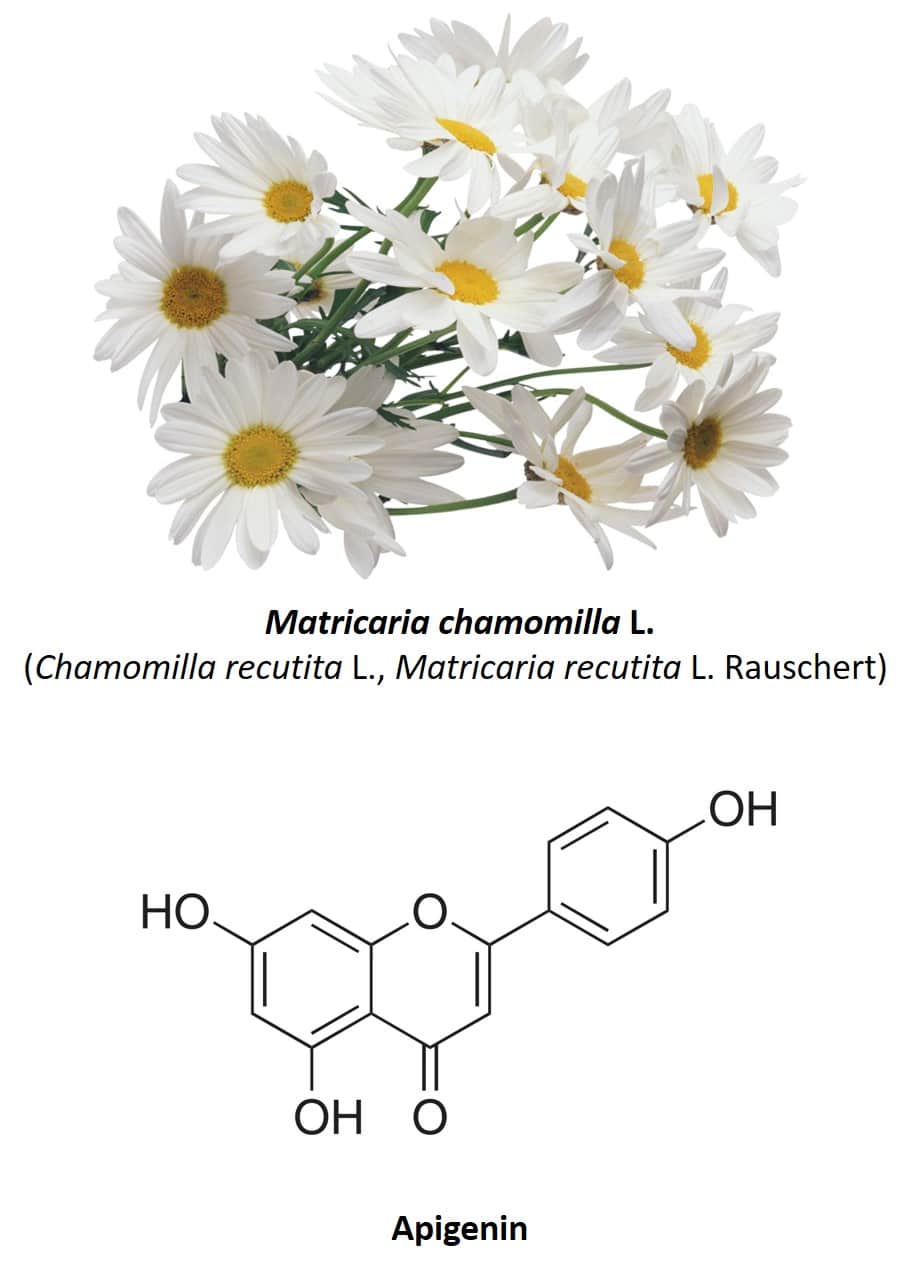
A characteristic of medicinal plants is the fact that they are almost always made up of a varied mixture of active ingredients, which means that the same plant species has several different medicinal properties. Chamomile or Matricaria chamomilla L. (also known as Chamomilla recutita or Matricaria recutita) (figure 4) is a clear example of this. This herbaceous plant native to Europe and Western Asia has several medicinal uses, including its use as anti-inflammatory, antibacterial, digestive, anxiolytic, antispasmodic, and anticonvulsant (30, 31). The most used part of the plant are its flowers, composed of several phenolic compounds, mainly the flavonoids apigenin, quercetin, patuletin, luteolin and its glycosides (31). Regarding the anxiolytic properties of chamomile, these are due to the presence of apigenin, a very abundant flavonoid which constitutes 68% of its total flavonoid content (32).
(click on the image to enlarge)
Figure 4: Chamomile flowers and apigenin chemical structure.
The anxiolytic properties of chamomile were first studied in 1982 by the group of Dr. Roberto DellaLoggia from the Faculty of Pharmacy of the University of Trieste (33). Using an animal model with rats, it was observed that the administration of an infusion of chamomile managed to exert a depressive action on the central nervous system of the animals, by reducing the basal rhythm and inducing sleep. It was also observed that the intensity of these effects depended on the concentration of the infusion used, which also corroborated that the anxiolytic effects observed came from the administration of chamomile. After this study, many others followed, but now with the aim of identifying the component of the plant responsible for this property. In 1995, Viola et al. Demonstrated that the chamomile flower contains several molecules capable of competitively binding to the same binding sites as benzodiazepines, notably apigenin, which exhibited clear anxiolytic activity in rats (34). In other words, this study suggests that apigenin might have benzodiazepine-like properties, by binding to the same binding sites used by benzodiazepines at GABA-A receptors. Subsequent studies indicate that the effects of apigenin on GABA-A receptors are even more complex and that other receptors may be involved in its anxiolytic effects (35–37). Most likely, apigenin acts as a second-order modulator of GABA-A receptors, in this case increasing the effect of a first-order modulator. The sedative and anxiolytic activities of apigenin observed in rats (34,35) can be interpreted based on apigenin enhancing the action of endogenous benzodiazepine-like agents in neurons (38, 39).
Due to the beneficial medicinal properties of chamomile flowers, the European Food Safety Authority (EFSA) is currently evaluating various health claims related to chamomile as its contribution to relaxation (EFSA-Q-2010-00538) and maintenance of healthy sleep (EFSA-Q-2010-00586).
In 4Sleep, each capsule (recommended daily dose) contains 110 mg of a concentrated extract (4:1) of chamomile, which is equivalent to consuming 440 mg of dried chamomile flowers per dose. This is a convenient and effective way to reap the benefits of chamomile.
Due to the beneficial medicinal properties of camomile flowers, the European Authority for Food Safety (EFSA) is evaluating several health declarations related to camomile as well as its contribution to relaxation (EFSA-Q-2010-00538) and for a maintenance of a sono saudável (EFSA-Q-2010-00586).
Valerian
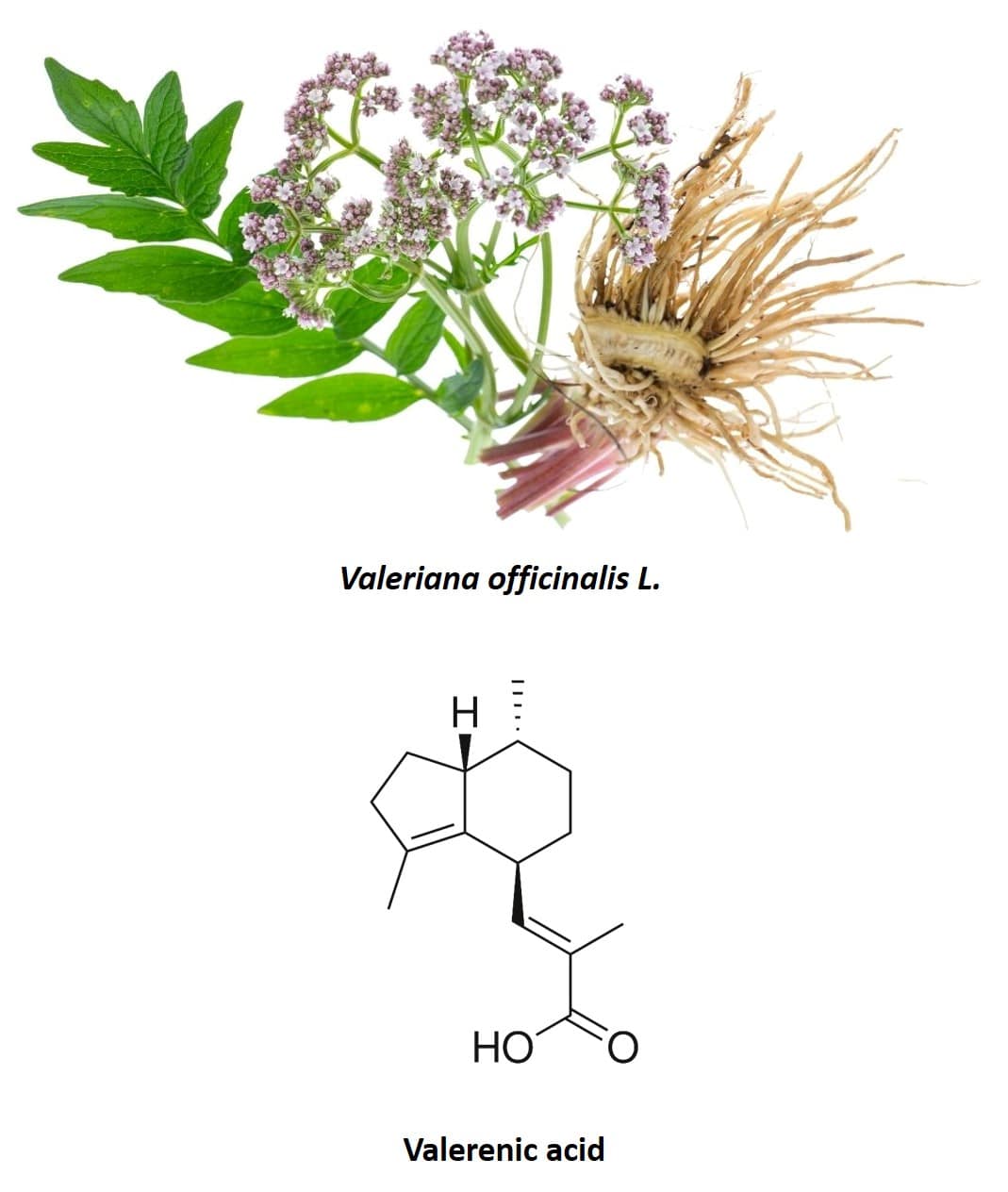
Valerian (Valeriana officinalis L.), a perennial plant native to North America, Asia, and Europe, is also another species traditionally used for its anxiolytic properties (40) (figure 5). In fact, it is one of the most widely used species and one of the most studied to verify its effects in the treatment of insomnia and other symptoms associated with nervousness. The most used part of the plant is its root, made up of several sesquiterpenoids and iridoids. The most important active compounds in valerian root are valepotriates (an iridoid) and valerenic acid (a sesquiterpenoid). Originally it was thought that only valepotriates were responsible for the sedative effects of valerian, however, purely aqueous extracts of valerian, such as those obtained in infusions, have also been shown to have a sedative effect (40, 41). As valepotriates are not soluble in water, it was concluded that valerenic acid present in valerian root also had anxiolytic properties and was the main chemical component responsible for the effect observed in human clinical trials. In addition to valepotriates and valerenic acid, the flavonoid 6-methylapigenin was isolated for the first time in 2002 and it was shown that this molecule was capable of binding to GABA-A receptors, producing an anxiolytic effect similar to that of benzodiazepines (42) . In the following years, the glycosides hesperidin (43) and linarin (44) were isolated, which are also capable of binding to GABA-A receptors, exhibiting sedative and hypnotic properties. Furthermore, the effect of hesperidin was synergistically enhanced by 6-methylapigenin and that of linarin by the valerenic acid present in the valerian composition.
(click on the image to enlarge)
Figure 5: Valerian root and chemical structure of valerenic acid.
Valepotriates showed cytotoxicity in in vitro studies, although this has not been demonstrated in vivo. Due to the high chemical instability of valepotriates, they are hardly found in commercial phytopharmaceutical preparations such as food supplements and, in addition, they have low absorption (45). In any case, the valerian extracts used in food supplements are obtained through a controlled mixture of water / bioethanol so as not to extract valepotriates.
4Sleep Valerian Extract is certified by accredited laboratories and contains no valepotriates. The requirement of this certificate is one of the criteria necessary for its commercialization in Europe.
From all components of valerian extract, the one that exerts the most important anxiolytic effect is valerenic acid (46). In addition, of all the substances identified in valerian that have this property, valerenic acid is the one that is present in the highest quantity in the plant. This substance also has the ability to modulate GABA-A receptors, producing a suppressive effect on neuronal activity. In this case, instead of binding to the gamma subunit as in the case of benzodiazepines, valerenic acid appears to bind to the beta subunit of the GABA-A receptor (47–50).
There are still few controlled clinical studies carried out with valerian, however, the available results suggest that its use facilitates the restructuring of the architecture of sleep after several weeks of treatment and, therefore, improves its quality. There is also evidence that it plays an important role in reducing anxiety stress in patients in whom this condition interferes with the initiation and maintenance of sleep, as well as in adjunctive treatment in the suspension of prolonged use of benzodiazepines (51).
EFSA is evaluating various health claims regarding this plant, for example: it helps to initiate sleep and maintain its quality (EFSA-Q-2008-3413, EFSA-Q-2008-4771) and supports mental well-being (EFSA-Q-2008-4932).
In 4Sleep, each capsule (recommended daily allowance) contains 110 mg of valerian root extract concentrate (3:1), which is equivalent to 330 mg of dried valerian root per capsule.
1. Chaput, J.-P., et al. (2018). “Sleeping hours: what is the ideal number and how does age impact this?” Nature and Science of Sleep 10: 421-430.
2. Hood, S. D., et al. (2014). “Benzodiazepine dependence and its treatment with low dose flumazenil.” British Journal of Clinical Pharmacology 77(2): 285-294.
3. Ngo, D.-H.; Vo, T. S. Molecules (Basel, Switzerland) 2019, 24, 2678.
4. Casati, A., et al. (2012). “Misuse of Medicines in the European Union: A Systematic Review of the Literature.” European Addiction Research 18(5): 228-245.Misuse of Medicines in the European Union: A Systematic Review of the Literature – FullText – European Addiction Research 2012, Vol. 18, No. 5 – Karger Publishers
5. Ladewig, D. (1983). “Abuse of benzodiazepines in western European society–incidence and prevalence, motives, drug acquisition.” Pharmacopsychiatria 16(4): 103-106.
6. Tan, K. R., et al. (2010). “Neural bases for addictive properties of benzodiazepines.” Nature 463(7282): 769-774.
7. Carlini, E. A. (2003). “Plants and the central nervous system.” Pharmacology Biochemistry and Behavior 75(3): 501-512.
8. Sarris, J., et al. (2011). “Re-introduction of Kava (Piper methysticum) to the EU: Is There a Way Forward?” Planta Med 77(02): 107-110.
9. Arendt, J. Reviews of reproduction 1998, 3, 13.
10. Karasek, M. Experimental Gerontology 2004, 39, 1723.
11. Costello, R. B., et al. (2014). “The effectiveness of melatonin for promoting healthy sleep: a rapid evidence assessment of the literature.” Nutrition Journal 13(1): 106.
12. Ferracioli-Oda, E., et al. (2013). “Meta-analysis: melatonin for the treatment of primary sleep disorders.” PLOS ONE 8(5): e63773.
13. Hunter, C. M.; Figueiro, M. G. Biological research for nursing 2017, 19, 365.
14. Rzepka-Migut, B.; Paprocka, J. Brain sciences 2020, 10.
15. Hartley, S.; Dauvilliers, Y.; Quera-Salva, M.-A. Current Neurology and Neuroscience Reports 2018, 18, 65.
16. Cagnacci, A. Climacteric 2017, 20, 183.
17. Chojnacki, C.; Kaczka, A.; Gasiorowska, A.; Fichna, J.; Chojnacki, J.; Brzozowski, T. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 2018, 69.
18. Tan, D.-X. (2010). “Melatonin and brain.” Current neuropharmacology 8(3): 161-161.
19. Zisapel, N. (2018). “New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation.” British Journal of Pharmacology 175(16): 3190-3199.
20. Andersen, L. P., et al. (2016). “The Safety of Melatonin in Humans.” Clin Drug Investig 36(3): 169-175.
21. Gooneratne, N. S., et al. (2012). “Melatonin pharmacokinetics following two different oral surge-sustained release doses in older adults.” Journal of pineal research 52(4): 437-445.
22. Brzezinski, A., et al. (2005). “Effects of exogenous melatonin on sleep: a meta-analysis.” Sleep Medicine Reviews 9(1): 41-50.
23. Buscemi N, et al. (2004). “Melatonin for Treatment of Sleep Disorders: Summary.” In: AHRQ Evidence Report Summaries. Rockville (MD): Agency for Healthcare Research and Quality (US); 1998-2005. 108.
24. Buscemi, N., et al. (2005). “The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis.” J Gen Intern Med 20(12): 1151-1158.
25. Buscemi, N., et al. (2006). “Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis.” BMJ (Clinical research ed.) 332(7538): 385-393.
26. Scientific Opinion on the substantiation of a health claim related to melatonin and reduction of sleep onset latency (ID 1698, 1780, 4080) pursuant to Article 13(1) of Regulation (EC) No 1924/2006 – 2011 – EFSA Journal.
27. Maffei, M. E. (2020) 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. International journal of molecular sciences 22.
28. Birdsall, T. C. (1998). “5-Hydroxytryptophan: a clinically-effective serotonin precursor.” Altern Med Rev 3(4): 271-280.
29. Zagajewski, J., et al. (2012). “Conversion L-tryptophan to melatonin in the gastrointestinal tract: the new high performance liquid chromatography method enabling simultaneous determination of six metabolites of L-tryptophan by native fluorescence and UV-VIS detection.” J Physiol Pharmacol 63(6): 613-621.
30. Srivastava, J. K.; Shankar, E.; Gupta, S. Mol Med Rep 2010, 3, 895.
31. McKay, D. L. and J. B. Blumberg (2006). “A Review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.).” Phytotherapy Research 20(7): 519-530.
32. Venigalla, M., et al. (2015). “Curcumin and Apigenin – novel and promising therapeutics against chronic neuroinflammation in Alzheimer’s disease.” Neural regeneration research 10(8): 1181-1185.
33. Loggia, R. D., et al. (1982). “Depressive effects of Chamomilla recutita (L.) Rausch, tubular flowers, on central nervous system in mice.” Pharmacological Research Communications 14(2): 153-162.
34. Viola, H., et al. (1995). “Apigenin, a Component of Matricaria recutita Flowers, is a Central Benzodiazepine Receptors-Ligand with Anxiolytic Effects.” Planta Med 61(03): 213-216.
35. Avallone, R., et al. (2000). “Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla.” Biochem Pharmacol 59(11): 1387-1394.
36. Salgueiro, J. B., et al. (1997). “Anxiolytic Natural and Synthetic Flavonoid Ligands of the Central Benzodiazepine Receptor Have No Effect on Memory Tasks in Rats.” Pharmacology Biochemistry and Behavior 58(4): 887-891.
37. Wasowski, C. and M. Marder (2012). “Flavonoids as GABAA receptor ligands: the whole story?” J Exp Pharmacol 4: 9-24.
38. Hanrahan, J. R., et al. (2011). “Flavonoid modulation of GABA(A) receptors.” British Journal of Pharmacology 163(2): 234-245.
39. Royal Society of New South, W. (2003). Dietary Chemicals and Brain Function. Journal and proceedings of the Royal Society of New South Wales. Sydney :, C. Potter, Acting. Govt. Printer. 135.
40. Houghton, P. J. (1999). “The scientific basis for the reputed activity of Valerian.” J Pharm Pharmacol 51(5): 505-512.
41. Andreatini, R., et al. (2002). “Effect of valepotriates (valerian extract) in generalized anxiety disorder: a randomized placebo-controlled pilot study.” Phytother Res 16(7): 650-654
42. Wasowski, C., et al. (2002). “Isolation and identification of 6-methylapigenin, a competitive ligand for the brain GABA(A) receptors, from Valeriana wallichii.” Planta Med 68(10): 934-936
43. Marder, M., et al. (2003). “6-Methylapigenin and hesperidin: new valeriana flavonoids with activity on the CNS.” Pharmacology Biochemistry and Behavior 75(3): 537-545
44. Fernández, S., et al. (2004). “Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis.” Pharmacology Biochemistry and Behavior 77(2): 399-404.
45. Villar Del Fresno, Á. M. and M. E. Carretero Accame (2001). “Valeriana officinalis. Fitoquímica, farmacología y terapéutica.” Farmacia Profesional 15(9): 98-107.
46. Benke, D., et al. (2009). “GABA A receptors as in vivo substrate for the anxiolytic action of valerenic acid, a major constituent of valerian root extracts.” Neuropharmacology 56(1): 174-181.
47. Khom, S., et al. (2007). “Valerenic acid potentiates and inhibits GABAA receptors: Molecular mechanism and subunit specificity.” Neuropharmacology 53(1): 178-187.
48. Becker, A., et al. (2014). “The anxiolytic effects of a Valerian extract is based on valerenic acid.” BMC Complement Altern Med 14: 267-267.
49. Trauner, G., et al. (2008). “Modulation of GABAA receptors by valerian extracts is related to the content of valerenic acid.” Planta Med 74(1): 19-24.
50. Das, G., et al. (2021). “Plant Species of Sub-Family Valerianaceae-A Review on Its Effect on the Central Nervous System.” Plants (Basel) 10(5).
51. Shinjyo, N., et al. (2020). “Valerian Root in Treating Sleep Problems and Associated Disorders—A Systematic Review and Meta-Analysis.” Journal of Evidence-Based Integrative Medicine 25: 1-31
Subscribe to our newsletter to save 5% on your first order.
This offer can be combined with all the other offers in the website.
Thank you!
You have successfully joined us. Please check your email with the discount code.